Introduction
The Rare Disease Treatment Market has been steadily transforming into one of the most crucial sectors in global healthcare. Rare diseases, often referred to as orphan diseases, are conditions that affect a very small percentage of the population. However, despite their low prevalence, they collectively impact millions of people worldwide. Moreover, the complexities involved in diagnosing, managing, and treating rare diseases have created an urgent demand for innovative therapies. In addition, as governments, healthcare providers, and pharmaceutical companies increasingly collaborate, the industry is witnessing unprecedented momentum.
Furthermore, the rise in genetic research, advancements in biotechnology, and the introduction of precision medicine are changing the way rare diseases are understood and treated. Therefore, the rare disease treatment market not only addresses a critical healthcare gap but also symbolizes hope for millions of patients and families. Consequently, it is an area of medicine that continues to attract investment, research interest, and global awareness.
Source: https://www.databridgemarketresearch.com/reports/global-rare-disease-treatment-market
The Evolution of the Rare Disease Treatment Market
In the beginning, rare diseases were largely overlooked due to their low patient populations and the perceived lack of commercial potential for treatments. Initially, most patients were left with limited care options, often relying only on symptomatic management rather than targeted therapies. However, as medical science advanced, the realization grew that genetic and molecular research could unlock targeted solutions even for very rare conditions.
Over the years, orphan drug legislation, policy incentives, and funding mechanisms dramatically changed the landscape. For instance, regulatory frameworks that encouraged pharmaceutical companies to develop treatments for rare conditions created an entirely new category of medicines called orphan drugs. Furthermore, the expansion of genomic sequencing and personalized medicine made it possible to better understand the molecular basis of rare conditions, leading to more precise therapeutic interventions.
Additionally, patient advocacy groups began playing a vital role in raising awareness, influencing policy, and funding research initiatives. As a result, the rare disease treatment market evolved from being neglected to being recognized as a vital and promising field within global healthcare. Consequently, this transformation has paved the way for innovative therapies such as gene therapy, enzyme replacement therapy, and targeted biologics.
Market Trends
Several significant trends are shaping the rare disease treatment market today. Firstly, there is a noticeable surge in the development of orphan drugs, which are specifically designed to treat rare conditions. Secondly, gene therapy and cell-based therapies are emerging as breakthrough solutions, providing the potential for long-term or even curative outcomes. Moreover, there is increasing emphasis on precision medicine, as treatments are increasingly being customized to target individual genetic profiles.
In addition, digital health technologies are becoming integral to rare disease management. Telemedicine platforms, artificial intelligence (AI)-driven diagnostic tools, and wearable health monitoring devices are now being used to improve early detection and patient outcomes. Furthermore, partnerships between pharmaceutical companies, academic research centers, and government bodies are accelerating innovation in treatment discovery and delivery.
Another key trend is the rising global awareness of rare diseases. As patient advocacy groups, awareness campaigns, and international observances such as Rare Disease Day gain traction, the spotlight on rare conditions continues to grow. Consequently, there is a stronger push for equitable healthcare policies and improved access to treatments across both developed and emerging markets.
Challenges
Despite the positive momentum, the rare disease treatment market faces numerous challenges. One of the most significant barriers is the high cost of therapies. Since patient populations are small, the research, development, and production costs for orphan drugs and advanced therapies are spread over fewer patients, making these treatments exceptionally expensive.
Additionally, delayed diagnosis remains a critical issue. Many rare diseases go undiagnosed or misdiagnosed for years due to limited awareness among healthcare providers and lack of specialized diagnostic tools. Furthermore, access to advanced treatments is uneven, with patients in low- and middle-income countries facing significant barriers to care.
Moreover, the regulatory landscape for rare disease therapies, while supportive in many regions, can still pose challenges. Variations in approval processes, reimbursement mechanisms, and intellectual property protections often create delays in market access. Another challenge is treatment adherence, as patients may face difficulties in sustaining long-term therapies due to costs, side effects, or complex treatment regimens.
Therefore, while the industry is making great strides, overcoming these barriers is crucial to ensuring that advancements truly benefit patients worldwide.
Market Scope
The scope of the Rare Disease Treatment Market extends across multiple therapy areas, healthcare delivery systems, and geographical regions. From a therapeutic standpoint, the market covers gene therapy, enzyme replacement therapy, biologics, small molecules, and symptomatic treatments. These therapies span a wide array of conditions, including genetic disorders, metabolic disorders, neurological conditions, hematologic diseases, and certain rare cancers.
From a distribution perspective, the market includes hospital pharmacies, specialty clinics, research hospitals, and retail or online pharmacies. Additionally, the role of clinical research organizations and academic institutions continues to expand, as they actively participate in drug development and clinical trials.
Geographically, North America and Europe dominate the rare disease treatment market due to robust healthcare infrastructure, strong regulatory support, and high R&D spending. Meanwhile, Asia-Pacific is emerging as a fast-growing region, fueled by rising awareness, government initiatives, and expanding access to healthcare. Moreover, Latin America, the Middle East, and Africa are increasingly contributing to global growth as healthcare systems in these regions modernize.
Thus, the scope of the rare disease treatment market is not confined to a few therapies or regions—it is global, multi-dimensional, and constantly expanding.
Market Size
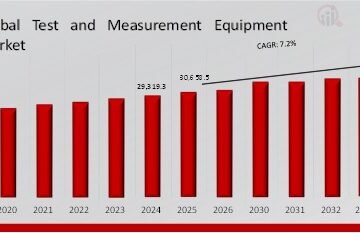
The global Rare Disease Treatment Market has grown significantly in recent years and is projected to expand even further. As the prevalence of rare diseases becomes more recognized and diagnostic capabilities improve, the number of identified patients continues to rise. Consequently, demand for effective treatments is growing at a strong pace.
Moreover, as pharmaceutical companies focus heavily on orphan drug development, market revenues are climbing steadily. The sector already represents a multi-billion-dollar global industry, and with the pipeline of therapies growing each year, the market size is expected to achieve double-digit growth in the coming decade.
Additionally, healthcare spending on rare diseases is increasing worldwide, supported by government programs, non-profit organizations, and private sector investments. Therefore, the combination of rising prevalence, expanding treatment pipelines, and increasing investment is pushing the rare disease treatment market toward sustained and robust growth.
Factors Driving Growth
Several factors are driving the growth of the rare disease treatment market. Firstly, the increasing prevalence of rare diseases worldwide ensures consistent demand for innovative therapies. Secondly, advancements in biotechnology, genomics, and precision medicine are enabling the development of highly targeted and effective treatments. Thirdly, government policies and orphan drug legislations are providing incentives to pharmaceutical companies to invest in this field.
Furthermore, the role of patient advocacy organizations cannot be overstated. These groups not only raise awareness but also influence funding decisions, accelerate clinical trials, and advocate for improved healthcare policies. Additionally, the rise of gene and cell therapies is creating transformative possibilities for rare disease patients, often offering long-term or curative benefits.
Moreover, the growth of digital healthcare platforms and AI-powered diagnostics is making early detection and ongoing patient management more efficient. Increasing collaboration between pharmaceutical companies, healthcare providers, and academic researchers is also accelerating innovation. Therefore, the combined effect of these scientific, regulatory, and societal factors is driving the rare disease treatment market toward unprecedented growth.
Conclusion
In conclusion, the Rare Disease Treatment Market is rapidly evolving into one of the most dynamic and impactful segments of global healthcare. While challenges such as high treatment costs, diagnostic delays, and access disparities remain, ongoing advancements in biotechnology, genomics, and precision medicine are steadily reshaping the landscape. Moreover, the growing involvement of patient advocacy groups, coupled with strong regulatory support and global awareness, is propelling the industry forward.
Consequently, the future outlook for the rare disease treatment market is highly promising. As innovation continues to flourish and access expands, patients worldwide will increasingly benefit from therapies that were once unimaginable. Therefore, the rare disease treatment market represents not only a business opportunity but also a powerful testament to the progress of modern medicine and the collective determination to address unmet healthcare needs.